Brexit and Covid‑19 are posing major legal challenges for the UK’s vibrant pharmaceutical industry, but the government is keeping lawyers guessing. Marialuisa Taddia reports
The low down
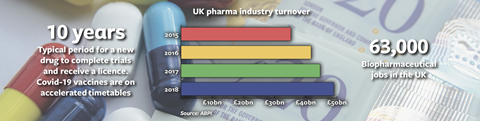
The development of Covid‑19 vaccines has made the regulation and licensing regimes of medicinal products headline news. How fast can justifiably strict regulatory machinery work to get a population safely vaccinated and back to work? Such is the significance of developments here that announcements are made with one eye on the science and the other on the stock market. Along with tech and logistics companies, pharma looks set to be among the pandemic’s ‘winners’. That prospect has been tempered by continuing uncertainty over a no-deal (or a ‘thin’ deal) Brexit and the implications of that for this £50bn UK industry. Less than four weeks from the end of the transition period, pharmaceutical companies and their lawyers have little idea where they stand.
As 2020 draws to an end Brexit and Covid‑19 are set to collide, with major implications for the UK. Both are strongly linked in the pharmaceutical and life sciences sectors, presenting unprecedented challenges and unmissable opportunities for UK-based legal practices.
Sally Shorthose, a Bird & Bird partner and IP specialist, says it is ‘impossible’ to mention UK pharma regulation without talking about Brexit. As the UK will no longer be under the auspices of the European Medicines Agency (EMA) – the EU’s medicinal products regulator – many companies have had to establish in the UK or the remaining member states in order to comply with the upcoming parallel regulatory regimes. In the event of a ‘no-deal Brexit’, these companies will have to apply to the EMA and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), part of the Department of Health and Social Care (DHSC), to get marketing authorisations that are valid in the EU and the UK, Shorthose says. As the Gazette went to press, time was running out to strike a deal.
'We have assisted clients trying to fast-track potential treatments, advised on the repurposing of factories to make PPE, and joined the race to find… a vaccine'
Sally Shorthose, Bird & Bird
‘This, plus the forthcoming application of tariffs and more bureaucratic processes with customs, has created considerable work and expense for pharma companies. The pharma industry was also charged with stockpiling in the event of a no-deal Brexit,’ she says.
‘At the same time, companies have been both dealing with and seizing opportunities that have arisen from the Covid‑19 pandemic. We have assisted clients trying to fast-track potential treatments, advised on the repurposing of factories to make PPE, and joined the race to find effective antibody tests and, of course – the Holy Grail – a vaccine,’ she adds.
The race for a vaccine gathered momentum in November. After compressing the usual decade-long process, US pharmaceutical giant Pfizer and the German biotechnology company BioNTech said they had completed phase 3 clinical trials for their vaccine (which precede the licensing of the product for general use) and are applying to the US Food and Drug Administration (FDA) for Emergency Use Authorization. This vaccine was last week approved by the MHRA for use in the UK. Other candidates include the vaccine developed by US biotech Moderna (which the firm claims has 94% efficacy in trials) and that developed by Oxford University and the UK’s AstraZeneca, which has met with controversy after two efficacy ratings were announced.
New rules for new drugs
With Brexit, the coronavirus crisis is the other event that is ‘fuelling’ regulatory change, notes Jonathan Turnbull. A partner in the disputes practice at Herbert Smith Freehills in London, Turnbull says that the MHRA and the government have reacted quickly ‘by strengthening the existing framework of regulatory law, instead of overhauling existing rules’. He argues that Covid‑19 ‘has stressed the need for more flexibility when dealing with regulation of time-sensitive, urgently required medicines, and has made regulators consider what changes can be made to their respective regimes to ease and shorten the regulatory process, without compromising any of the safety, efficacy and quality requirements that regulatory law is intended to safeguard’.
Evidence of this comes in amendments to the Human Medicines Regulations 2012 to support the roll-out of Covid‑19 and flu vaccines. The Human Medicines (Coronavirus and Influenza) (Amendment) Regulations 2020 came into force on 16 October, following a public consultation held by the DHSC between 28 August and 18 September. The department’s response, issued on the day the statutory instrument became law, said the changes will ‘strengthen existing regulations that allow for the temporary licensing of medicines and vaccines, on an exceptional basis, pending the grant of a full licence’ and will also ‘extend the current immunity from civil liability to companies producing the vaccine, rather than just healthcare workers and manufacturers. This will protect them from legal liability in civil cases, although it would ‘not give [companies] blanket immunity from civil liability’.
In its consultation document, the DHSC said the ‘preferred route’ to deploy a Covid‑19 vaccine is through the usual marketing authorisation or product licensing process. ‘However, if there is a compelling case, on public health grounds, for using a vaccine before it is given a product licence, given the nature of the threat we face, [the independent Joint Committee on Vaccination and Immunisation] may take the very unusual step of advising the UK government to use a tested, unlicensed vaccine against Covid‑19, and we need to make sure that the right legislative measures are in place to deal with that scenario.’
The UK is not the only country to boost pharmaceutical companies’ indemnity. In April, under the Public Readiness and Emergency Preparedness Act 2005, the US extended liability immunity for ‘activities related to medical countermeasures against Covid‑19’, defined as ‘any antiviral, any other drug, any biologic, any diagnostic, any other device, any respiratory protective device, or any vaccine, used to treat, diagnose, cure, prevent, or mitigate Covid‑19, or the transmission of SARS‑CoV‑2 [the official name of the coronavirus] or a virus mutating therefrom, or any device used in the administration of any such product, and all components and constituent materials of any such product’.
Phelim O’Doherty, a partner in Baker McKenzie’s corporate practice in London, does not expect the acceleration of the development of Covid‑19 treatments and vaccines ‘to become the norm’.
‘Ensuring medicine’s long-term safety is as important as rapid approval of new treatments,’ he says. ‘Regulators worldwide have made clear that the procedural flexibilities that have been introduced are aimed at ensuring the safety of patients during these unprecedented times.’
But many trends that have accelerated as a result of Covid‑19, such as digitalisation or the greater focus on ensuring availability of medicines and the prevention of shortages, ‘will remain beyond the pandemic’, he said.
‘The pandemic has been touted as the opportunity for a “digital reset” and there is great potential for pharmaceutical companies to implement remote monitoring tools, as well as educational and information tools for their patients,’ O’Doherty says. At the same time, he says, this creates ‘regulatory challenges’ regarding the use and protection of personal data, market access and promotion of medicines, given the decrease in personal interaction between sales representatives and healthcare professionals.
The World Health Organization and the World Intellectual Property Organization (WIPO) are expected to collaborate to enable wider access to some patented drugs and medical supplies during the pandemic, Shorthose says. In April, Francis Gurry, WIPO’s director general, said that during an emergency, health and safety ‘trumps everything’ and that extraordinary situations call for ‘extraordinary measures’. ‘A very specific compulsory license on a very specific product to ensure the supply of product in the market’ is ‘arguably the sort of action that we need’. Shorthose says she would not be surprised to see a ‘coronavirus patent pool before long’.
Jane Summerfield, co-head of Hogan Lovells’ life sciences and health care practice, refers to ‘regulatory flexibilities’ introduced by the UK’s medicines regulator to ‘assist the smooth functioning and operation of the pharmaceutical industry’. For example, the MHRA has established a fast-track application process for clinical trials for coronavirus treatments and tests. Under this new process, initial applications for reviews and approvals take an average of nine days. She also highlights ‘a marked increase’ in off-label use of some products and, in some cases, the use of unlicensed drugs to treat Covid‑19.
Fighting talk
The increased use of diagnostic testing has seen a corresponding growth in patent litigation (for example, the various proceedings in the High Court’s Patents Court brought by US firm Illumina) along with a trend of litigation surrounding biological products and the use of complex medical devices, according to Herbert Smith Freehills partner Jonathan Turnbull.
As biological products are more complex to develop and manufacture, this has led to more patent disputes between R&D-based pharmaceutical companies (eg, Regeneron v Kymab, which was heard before the UK Supreme Court in February) and between makers of ‘biosimilars’ of the same drug, according to Turnbull.
Commenting on the increase in judicial proceedings around biosimilars, Andrew Bowler, partner and head of the patent litigation department at Bristows, refers to ‘the use of Arrow declarations, where the biosimilar entrant seeks a declaration that the patented product lacked novelty or was obvious at a particular date [or both] in order to provide it with commercial certainty in relation to a patentee’s patent portfolio’ (eg, Pfizer v Hoffmann–La Roche).
Patent litigators have also been busy with cases dealing with the extent to which judges should exercise their discretion to grant injunctive relief when an infringing product has life-saving or life-changing benefits for patients, Bowler notes. An example is Evalve v Edwards Lifesciences, a heart valve patent case in the High Court.
The NHS appears to be more of a protagonist in litigation, particularly where it is able to claim damages for wrongly awarded preliminary injunctions that have prevented it purchasing generic products, Turnbull notes (such as the NHS’s recent claims against the US pharma company Warner–Lambert). But the NHS is also facing challenges to the legality of some of its policies, including litigation initiated by Bayer concerning the NHS’s policies around the ‘off-label’ use of drugs.
Speedy approvals
In its most recent guidance, published at the end of October, the MHRA announced changes to the UK’s licensing procedures from 1 January 2021, including an ‘accelerated assessment procedure’ for obtaining marketing authorisation in the UK and a ‘rolling review’.
The accelerated procedure will allow applicants to obtain marketing authorisation in 150 days instead of the usual 210, Turnbull observes, while the ‘rolling review’ aims to enhance the development of novel medicines ‘by offering ongoing regulatory input and feedback enabling the applicants to “get it right first time”’.
In the pharmaceutical world, Brexit and Covid‑19 are closely linked by the MHRA. ‘Come January 2021, all eyes will be on the MHRA. It is an opportunity not just for the MHRA to carve a niche for itself, but also for UK-based practices to do the same,’ says Turnbull.
'The MHRA has expressed its desire to, at least in the short-term, maintain as much regulatory continuity with the EU as possible'
Jane Summerfield, Hogan Lovells
Until 31 December, EU legislation requires biotechology medicines, including candidate Covid‑19 vaccines, that are to be used in the EU to be authorised by the EMA. Currently, EMA marketing authorisation is valid in the UK. But from 1 January 2021, the MHRA will be responsible for licensing all medicines used in the UK, including vaccines.
Summerfield says that if the UK and EU fail to agree a trade deal, a mutual recognition agreement (MRA) or equivalent, the EU will treat the UK as a ‘third country’, ending the UK’s involvement with the EU’s regulatory network and access to its shared databases and processes. ‘The MHRA will become a standalone regulator and will need to perform tasks which were previously performed at the EU level,’ she says.
Summerfield says the MHRA does not want this. ‘Throughout the Brexit negotiations, the MHRA has expressed its desire to, at least in the short-term, maintain as much regulatory continuity with the EU as possible to afford industry greater certainty,’ she says.
O’Doherty says: ‘In the absence of a trade deal, industry trade bodies in the EU [the European Federation of Pharmaceutical Industries] and in the UK [the Association of the British Pharmaceutical Industry (ABPI)] have called for at least technical [MRAs] for medicine manufacturing.’
Iain MacVay, a partner in White & Case’s global international trade practice, tells the Gazette that an MRA ‘between the UK and the EU, along the lines of similar MRAs in place for a number of jurisdictions, would be a very important first step to support the industry in meeting the new challenges of moving pharmaceutical products between the UK and EU jurisdictions after 1 January 2021’.
Following the fourth meeting of the Ireland/Northern Ireland Specialised Committee on 5 November, the UK and the European Commission did agree to a phased process for implementing medicines regulation in Northern Ireland until 31 December 2021. ‘This decision is very welcome, as it will allow time for government authorities and businesses to prepare for full implementation of rules on batch testing, importation and counterfeit medicines,’ MacVay says.
The ABPI has urged both sides to use the rest of the transition period to ‘clarify the rules which will apply in Northern Ireland from 2022, so that companies can make full use of this extra time to prepare for the long-term’. Under the NI Protocol, medicines in Northern Ireland will be governed by EU regulations that will be enforced by the MHRA. ‘Both sides need to agree how the regulations will be interpreted and implemented come December 2021,’ the ABPI said.

In November, a petition by the ABPI and the BioIndustry Association led the government to change its position on when protection will begin for a number of ‘regulatory exclusivities’ (a form of IP right specific to pharmaceutical products) after Brexit. These changes are to be incorporated in another statutory instrument, currently in its draft form, called the Human Medicines (Amendment etc) (EU Exit) Regulations 2020, which is due to take effect on 1 January. Further, in October, the DHSC announced that the UK had joined two international collaboration schemes – the FDA’s Project Orbis and the Australia–Canada–Singapore–Switzerland (ACSS) Consortium – to fast track the approval of innovative medicines.
For Turnbull, both the MHRA guidance and the Medicines and Medical Devices Bill 2019-21 ‘reflect the government’s vision for a post-Brexit Britain […] which positions the MHRA as a leading regulator and the UK as a competitive, sophisticated market’.
The bill, sponsored by health secretary Matt Hancock and minister for innovation Lord Bethell of Romford, started in the House of Commons on 13 February and is currently at report stage in the House of Lords. It attempts to fill the regulatory gap that will be left at the end of the transition period, as the regulation and licensing of medicinal products in the UK emanates from the EU. Lord Bethell has described the new medicine regulation regime as ‘absolutely essential to protect the lives of patients and empower the innovations that will extend their length and quality’.
‘[It] is a crucial highlight because it gives the secretary of state very broad powers to change the existing law to make the UK a more attractive place to develop and commercialise medicines,’ says Bristows partner Alex Denoon. But he adds that ‘how this will happen in practice is not very clear yet, and the whole industry is waiting to know’.
Denoon notes that the bill was criticised by Lord Blencathra, chair of the Delegated Powers and Regulatory Reform Committee, in the House of Lords, for being ‘absolutely atrocious and an affront to parliamentary democracy’ containing ‘inappropriate delegations of power’.
Blencathra, a Conservative life peer and former MP, said: ‘Of course, it is not unique; it is just one more bill stuffed full of Henry VIII clauses but devoid of substantive content. It is the barest skeleton, all to be filled in with negative secondary legislation.’
Whether or not the UK will fill the void left by EU law and how far its regulatory framework will diverge are big questions. And the repercussions of Brexit do not just impact the UK. ‘The MHRA is a world-leading regulator,’ says Denoon. Its ‘absence from the grown-ups’ table at the European level severely weakens the EU regulatory framework’.
Marialuisa Taddia is a freelance journalist
































No comments yet